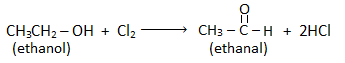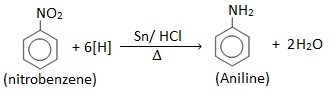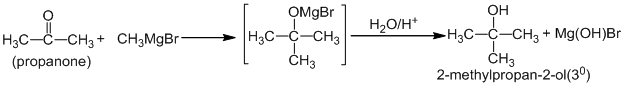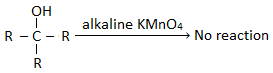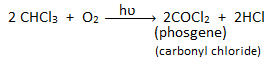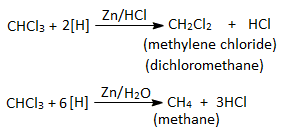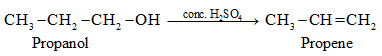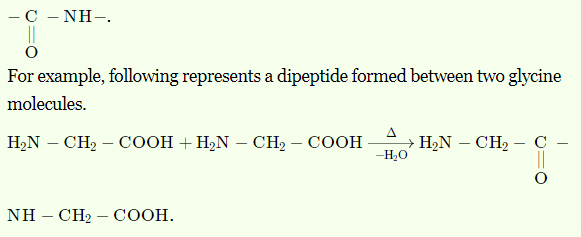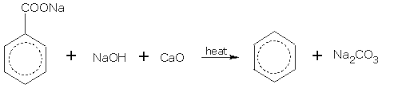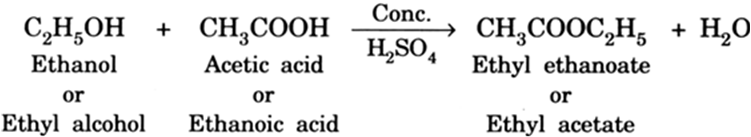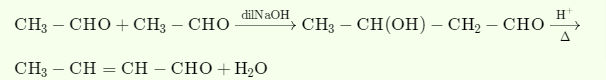Lab preparation of Chloroform (trichloromethane )
Principle:
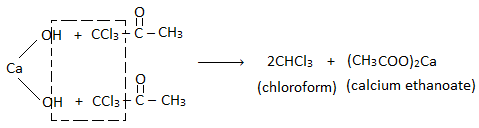
Chloroform(trichloromethane ) is prepared in the laboratory by heating ethanol or acetone with aqueous bleaching powder paste. In this process, bleaching powder paste acts as oxidizing, chlorinating, and hydrolyzing agent.
From ethanol :
Step I: Oxidation :
Step II: Chlorination :
Step III: Hydrolysis :
From acetone (propanone) :
Step I : Chlorination :
Step II: Hydrolysis :
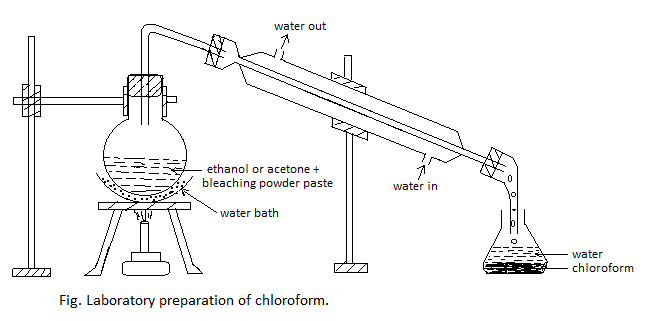
Procedure: First of all, bleaching powder paste is prepared by mixing 100 gm of bleaching powder with 200 ml of water in one liter round-bottomed flask and 25 ml of ethanol or acetone is added to it. The flask is heated gently on a water bath until a mixture of chloroform and water distills over, as shown in the figure. The mixture from the receiver is transferred into a separating funnel and the lower layer of chloroform is separated.
Purification: The impure chloroform is washed with dilute caustic soda (NaOH) solution and then with water successively in the separating funnel. It is then dried over anhydrous calcium chloride and re-distilled between 60 – 650C. In this way, pure and dry chloroform is obtained.
Laboratory preparation of diethyl ether (ethoxyethane)
Principle:
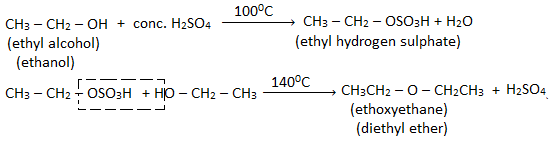
When an excess of ethyl alcohol is heated with conc. H2SO4 at 1400C, diethyl ether is obtained.
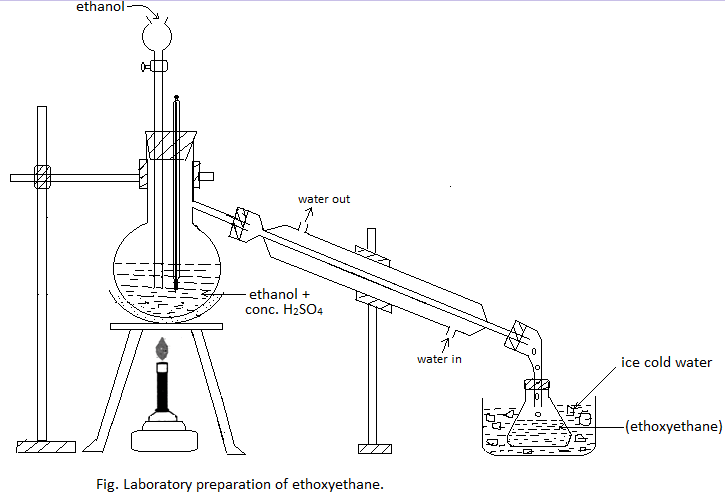
Procedure: A mixture of ethyl alcohol and conc. H2SO4 in the ratio of 1:1 by volume is taken in the distillation flask. The flask is then fitted with a dropping funnel containing alcohol. The flask is heated on a sand bath at 1400C. Ethanol is added at nearly the same rate as that of the distillation so that the ether formed is continuously received in the receiver kept cold in the ice-cold water.
Purification: The distillate thus obtained contains ether, ethyl alcohol, water, and sulphurous acid. The acid is removed by washing with KOH or NaOH solution. The solution is then stirred with anhydrous CaCl2 to remove the alcohol. Finally, it is redistilled to obtain almost pure ether.
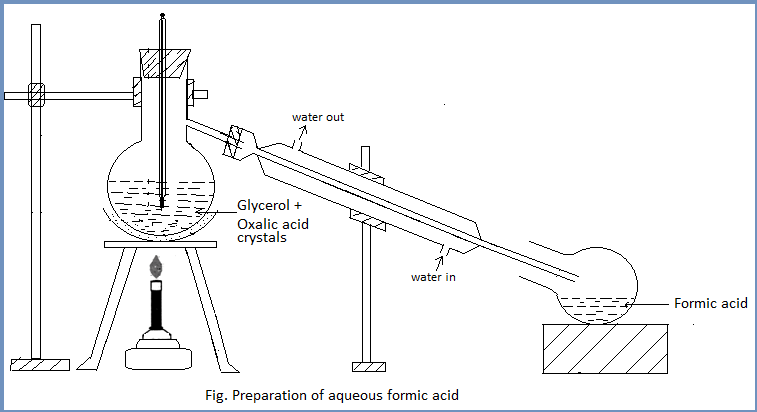
Laboratory preparation of anhydrous formic acid (methanoic acid)
Principle:
Formic acid is prepared in the laboratory by the decarboxylation of oxalic acid with glycerol at 1100C.
This reaction occurs in the following steps:
Procedure: About 50ml of anhydrous glycerol and 40gm of oxalic acid crystals are placed in a flask and all the apparatus are fitted as shown in the figure. The flask is heated at 1100C till the evolution of carbon dioxide (Marked by effervescence) ceases. The reaction flask is then cooled and a fresh lot of oxalic acid (40gm) is added. The mixture is again heated at 1100C and the distilled aqueous solution of formic acid is collected in the receiver.
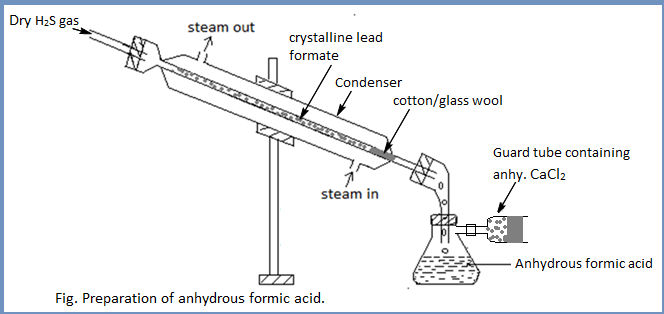
For anhydrous formic acid:
The aqueous formic acid is neutralized with lead carbonate and the lead formate solution is then evaporated to get crystals of lead formate. The dry lead formate is packed in the inner tube of the condenser and treated with dry H2S gas. As a result anhydrous formic acid is formed which is collected in the receiver.
Anhydrous formic acid so obtained contains traces of H2S. it is mixed with some lead formate and is distilled to obtain pure formic acid.
Note: Anhydrous HCOOH can’t be obtained by distillation of aqueous HCOOH as b.pt. of HCOOH (b.pt. 100.50C) is almost similar to that of water.
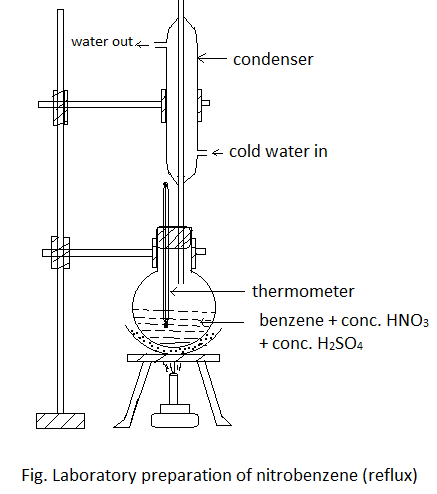
Laboratory Preparation of Nitrobenzene
Principle:
It is prepared in the lab by heating benzene with conc. HNO3 and conc. H2SO4 at 600C.
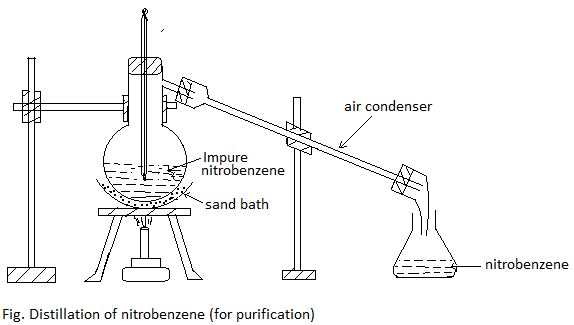
Procedure: 50 ml of benzene is taken in a round-bottomed flask. To this flask, 60 ml conc. HNO3 and 60 ml conc. H2SO4 (i.e. nitrating mixture) is added a little at a time, shaking and cooling after each addition. Then the mixture is heated (refluxed) in a water bath at 600C for about one and half hours till the yellow oily layer appears on the surface. The flask is then cooled and the layer of nitrobenzene is separated by using a separating funnel.
Purification: It is first washed with dil. Na2CO3 to remove the acidic impurities and then with water several times. It is then dried over fused calcium chloride. It is finally distilled at 2110C to get pure nitrobenzene.
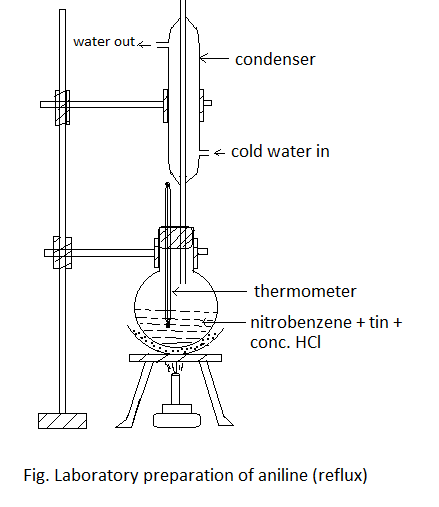
Laboratory preparation of aniline
Principle:
Aniline is prepared in the laboratory by reducing nitrobenzene with tin (Sn) and conc. HCl.
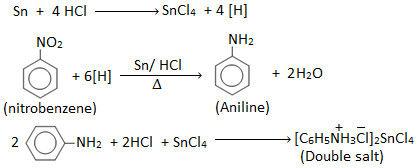
Procedure: 10 ml nitrobenzene and 20 gm of granulated tin are placed in the 250 ml round bottom flask fitted with a reflux condenser. 50 ml of conc. HCl is added gradually with constant shaking. After each addition, the round bottom flask is cooled so that the temperature may not go above 900C. Then the reaction mixture is heated on a boiling water bath for about one hour until the reaction is completed which is indicated by the smell of nitrobenzene, the disappearance of smell indicates the completion of the reaction. The flask is then cooled and a crystalline solid mass of double salt is separated out.
The crystalline solid mass is then treated with conc. NaOH until the solution is cleared and becomes strongly alkaline. Aniline is separated out and is floated on the surface as a dark brown oil.
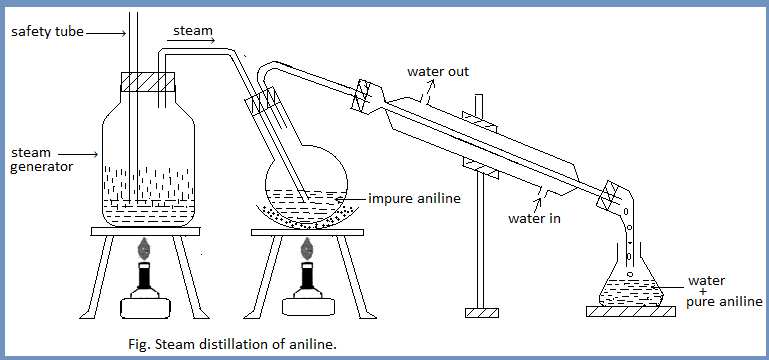
The mixture obtained is then subjected to the process of steam distillation until clear distillate is obtained.
Purification: Aniline is extracted by shaking the distillate several times with ether. The ethereal layer is separated each time with the help of a separating funnel. Now, the ethereal aniline is placed for the evaporation where ether evaporates out. Aniline thus obtained is finally purified by redistillation at 182-1840C.
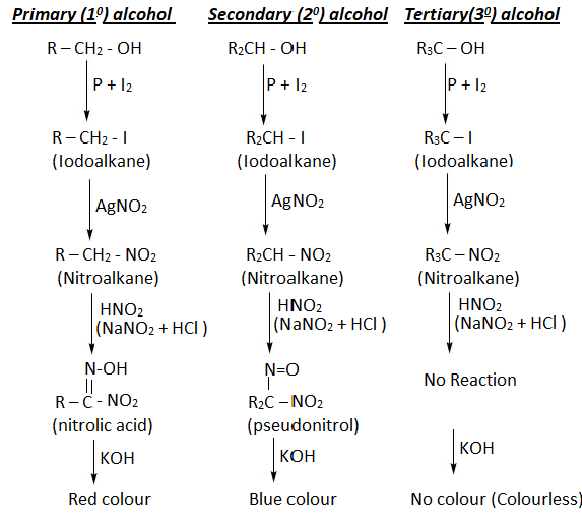
- Identification of 1°, 2°, and 3° alcohols by Victor Meyer's method
- Separation of 1°, 2°, and 3° amines by Hoffmann's method
➵
In this method given alcohol is treated with different chemicals and involves the following steps:-
Step 1:- The given alcohol is treated with a mixture of Phosphorous & Idodine(Red P & I2 )
Step 2:- The ido alkane is then treated with silver nitrate(AgNo2) solution then corresponding nitro alkane is formed.
Step 3:- The nitro alkane is then treated with nitreous acid(HNO2)
Step 4:- The resulting solution is made alkaline with aq. NaOH or KOH.
*If blood blue coloration is observed then the given alcohol is secondary(2°) alcohol.
*The colorless solution represents the tertiary(3°) alcohol.
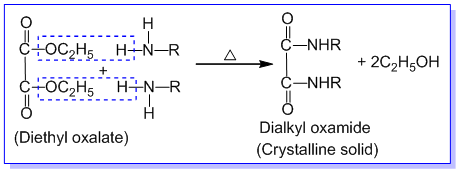
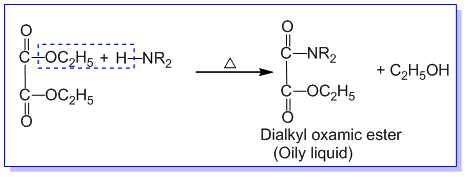
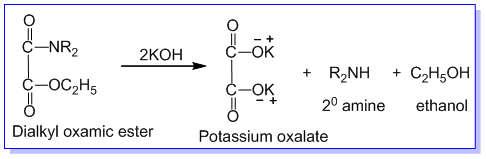
➵The mixture of three amines is treated with diethyl oxalate. The primary amine forms a solid oxamide, a secondary amine gives a liquid oxamic ester while tertiary amine does not react.
1) 1° amine forms dialkyl oxamide which is a crystalline solid.
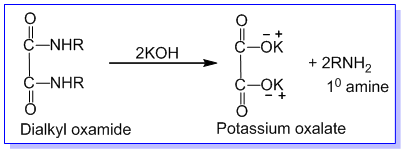
The reaction mixture containing dialkyl oxamide, dialkyl oxamic ester, tertiary amine, and ethyl alcohol is first filtered and the solid product of dialkyl oxamide is separated. Dialkyl oxamide is heated with aq.KOH to recover primary amine. The remaining mixture of dialkyl oxamic ester, tertiary amine, and ethyl alcohol is subjected to fractional distillation. The tertiary amine is distilled out first. The residual dialkyl oxamic ester is heated with aq. KOH to recover secondary amine and alcohol in different fractions. In this way, the given mixture of 1°,2°, and 3° amines are separated by Hoffmann’s method.
- Perkins condensation
- Claisen Condensation reaction.
- Benzoin condensation
- Diazotization reaction
- Esterification reaction
- Carbonylation reaction
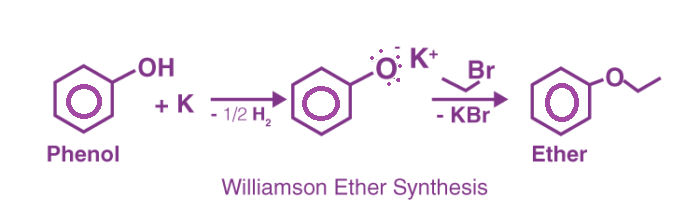
- Williamson's etherification reaction
The Perkin reaction, also known as Perkin condensation, is an organic reaction that is used for the synthesis of α,β-unsaturated aromatic acid by the condensation of an aromatic aldehyde and an acid anhydride, in the presence of an alkali salt of the acid acting as a weak base.
C6H5CHO + (CH3CO)2O→ C6H5CH = CHCO2H
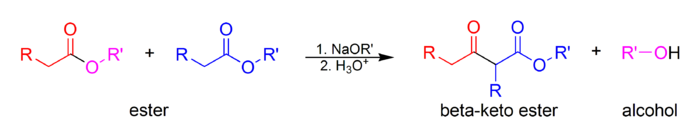
The Claisen condensation is a carbon-carbon bond-forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone.
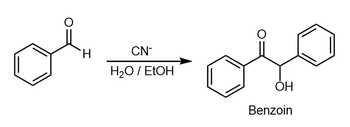
The benzoin addition is an addition reaction involving two aldehydes. The reaction generally occurs between aromatic aldehydes or glyoxals. The reaction produces an acyloin. In the classic application, benzaldehyde is converted to benzoin.
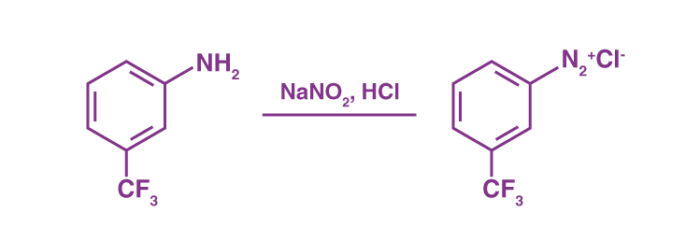
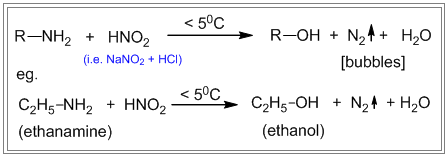
The chemical process used in converting a primary aromatic amine into the corresponding diazonium salt of the amine is commonly referred to as diazotization. This process is also known as ‘diazotization’.
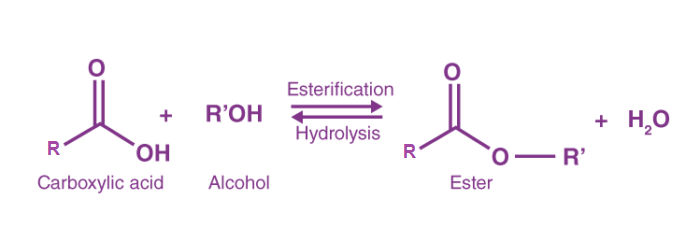
Esterification is the process of combining an organic acid (RCOOH) with an alcohol (ROH) to form an ester (RCOOR) and water; or a chemical reaction resulting in the formation of at least one ester product. Ester is obtained by an esterification reaction of an alcohol and a carboxylic acid.
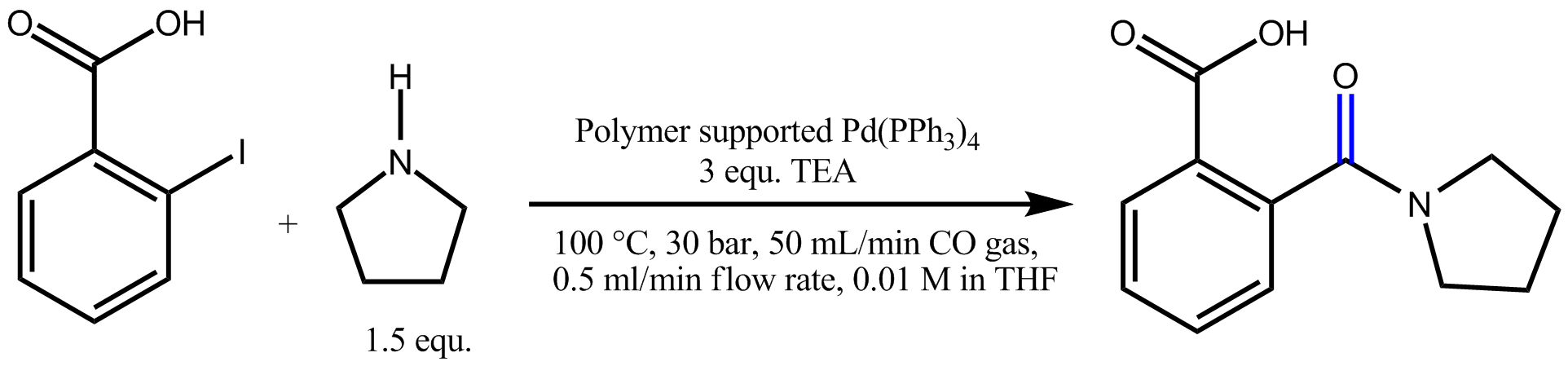
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates.
The general method for the synthesis of ether is Williamson ether synthesis, which involves nucleophilic displacement of a halide ion or other good leaving group by an alkoxide ion.
- Iodoform test ( NaOH + I2)
- Fehling's test ( alkaline solution of copper sulphate)
- Silver mirror test( reaction with Tollens reagent)
- The Nitrous acid test of 1º, 2º, and 3° amines Primary, secondary and tertiary amines react differently with nitrous acid.
- 2,4 – DNP test
- Carblyamine test ( test of primary amines)
Fehling's test is a chemical test used to differentiate between reducing and non-reducing sugars. This test can also be used to differentiate between carbohydrates and liquid carbohydrates in the ketone functional community.
Reactions of Fehling’s Test
The response between copper(II) ions and an aldehyde is expressed in Fehling's solution as:
RCHO + 2Cu2+ + 5 OH- → RCOO- + Cu2O + 3 H2O
Once tartrate has been added,
RCHO + 2 Cu(C4H4O6)22−+ 5 OH− → RCOO− + Cu2O + 4 C4H4O62− + 3 H2O
It is a qualitative laboratory test which is used to distinguish between an aldehyde and a ketone. The reagent used for this test is Tollens’ reagent which has silver ions coordinated to ammonia ions. The solution is colourless initially.
Step 1:- The aqueous silver nitrate is taken in the test tube. Added aqueous sodium hydroxide solution to it. The reaction for this step is
AgNO3+NaOH→AgOH+NaHO3
2AgOH→Ag2O+H2O
Step 2:- To this precipitate of Ag2O (silver oxide), aqueous ammonia is added dropwise till we get this ppt completely dissolved.
Ag2O+4NH3+H2O→2Ag(NH3)2++2OH−
Step 3:- These hydroxide ions produced in step 2 are used to oxidize the group. If the compound given is aldehyde; it readily gets oxidized while if the compound contains a ketonic group; then it is not hydrolyzed.
CH3CHO+2Ag++2OH−→CH3COOH+2Ag+H2O
Further, the silver ions are reduced into metallic silver. This metallic silver forms a mirror in a clean test tube. Thus, the test is named the silver mirror test.
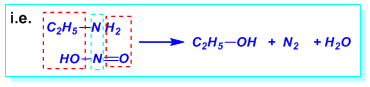
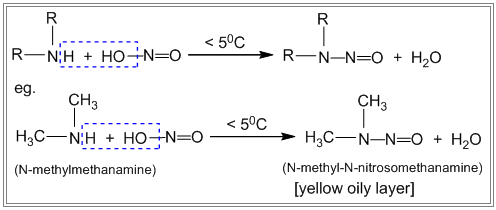
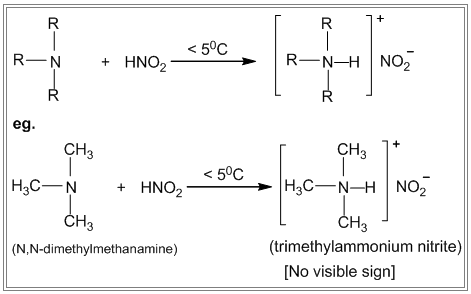
i. Primary amines react with nitrous acid to produce nitrogen gas which is seen as bubbles. ii. Secondary amines react with nitrous acid to produce a yellow oily layer of N-nitrosamine. iii. Tertiary amines react with nitrous acid to form soluble trialkyl ammonium nitrite salt. There is no visible sign of reaction.
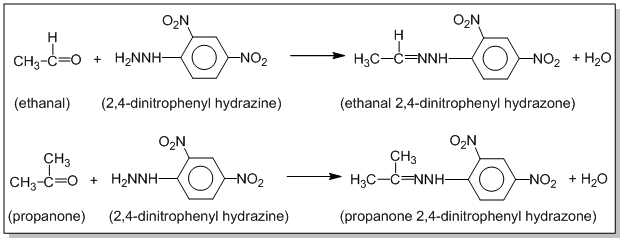
Aldehydes and ketones react with 2,4-dinitrophenyl hydrazine (2,4-DNP) to form yellow, orange, or red ppt. of 2,4-dinitrophenyl hydrazone. Eg.
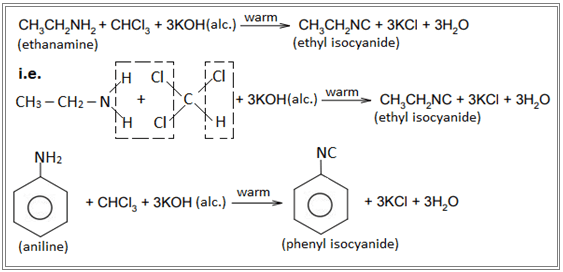
When primary amine is warmed with chloroform in the presence of alcoholic KOH, an offensive (unpleasant) smell of carbylamines ( i.e. isocyanide) is obtained. This reaction is known as carbylamine reaction. Eg.
Secondary and tertiary amines do not respond to this reaction and therefore, this reaction is used as a test reaction for primary amines.
- Reduction of nitrobenzene in a different medium. Nitrobenzene gives different products in different mediums by using a different reducing agent.
- Reduction of nitrobenzene in acidic medium :
- Catalytic reduction of nitrobenzene :
- Reduction of nitrobenzene in neutral medium :
- Reduction of nitrobenzene with LiAlH4 :
- Reduction of nitrobenzene in alkaline (basic) medium :
- Electrolytic reduction of nitrobenzene :
- Preparation of alcohol using a Grignard reagent. C3H8 and C4H10
- Oxidation of alcohols. ( 1, 2 & 3 degree) Alcohols are oxidized by different oxidizing agents like acidic or alkaline KMnO4, acidified K2Cr2O7, dil. HNO3, etc. to give different products.
- Fermentation
- 2,4 – DNP test
- All reactions of chloroform. 1. Reaction with air : Oxidation of chloroform
Nitrobenzene on reduction with Zn/HCl or Sn/ HCl gives aniline.
Nitrobenzene when reduced by hydrogen in presence of nickel or platinum as a catalyst gives aniline.
Nitrobenzene on reduction with Zn and aq. NH4Cl gives phenylhydroxylamine.
Lithium aluminium hydride reduces nitrobenzene to azobenzene.
Nitrobenzene when reduced electrolytically, first gives phenylhydroxylamine which immediately rearranges to give p- aminophenol.
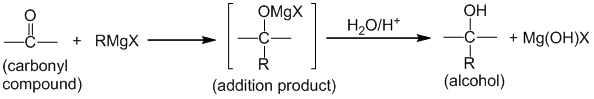
Aldehydes and ketones (i.e carbonyl compounds) when treated with Grignard reagent gives additional product, which upon acidic hydrolysis give alcohols
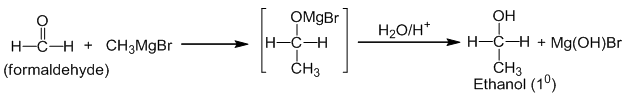
a. Formaldehyde gives primary alcohol. Eg.
b. Aldehydes other than formaldehyde give secondary alcohol. Eg.
c. Ketones give tertiary alcohol. Eg.
i. Primary alcohols are easily oxidized first to aldehyde and then to carboxylic acids containing the same number of C- atoms as in parent alcohol. Eg. ii. Secondary alcohols on oxidation give ketones with the same number of carbon atoms. The ketones are further oxidized only under drastic conditions ( i.e. prolong treatment of oxidizing agent) to give carboxylic acid-containing lesser number of carbon atoms. iii. Tertiary alcohols do not contain hydrogen atoms on the carbon carrying – OH group (i.e. α- hydrogen). Thus in order to oxidize tertiary alcohol, a carbon-carbon bond must be broken. For this reason, 30 alcohol do not undergo oxidation reaction in neutral or alkaline medium. But if the oxidation is carried out in the acidic medium under drastic conditions tertiary alcohol oxidize to give a mixture of ketone and carboxylic acid. The ketone thus formed further gets oxidized to carboxylic acid.
Aldehydes and ketones react with 2,4-dinitrophenyl hydrazine (2,4-DNP) to form yellow, orange or red ppt. of 2,4-dinitrophenyl hydrazone. Eg.
In the presence of sunlight, chloroform is oxidized by air to produce a highly poisonous gaseous compound called phosgene (carbonyl chloride). Thus, chloroform is stored in a dark/colored bottle to prevent the oxidation of chloroform into phosgene.
A small amount of ethanol is also added into the chloroform at the time of packing because ethanol converts highly poisonous phosgene ( if formed) to a non-poisonous diethyl carbonate. 2. Reaction with aq. KOH solution :
When boiling with aqueous KOH solution, chloroform is hydrolyzed to form potassium formate which on acidification gives formic acid. 3. Reaction with silver powder :
Chloroform when heated with silver powder gives acetylene (ethyne). 4. Reaction with primary amines ( carbylamine reaction) :
When chloroform is warmed with a primary amine in the presence of alcoholic KOH, an offensive(unpleasant) smell of carbylamines ( i.e. isocyanide) is obtained. This reaction is known as carbylamine reaction.
Note : Secondary and tertiary amines do not respond to this reaction and therefore, this reaction is used as test reaction for primary amines.
5. Reaction with phenol (Riemer- Tiemann reaction) :
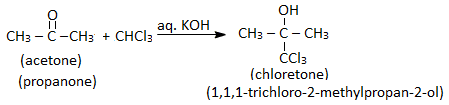
When chloroform is heated with phenol and sodium hydroxide followed by hydrolysis, o – hydroxy benzaldehyde ( salicylaldehyde) is formed. This reaction is called Riemer – Tiemann reaction. 6. Reaction with acetone (propanone) :
Chloroform reacts with acetone in presence of a base such as KOH to give chloretone. Chloretone is used as a sleep-inducing (hypnotic) drug.
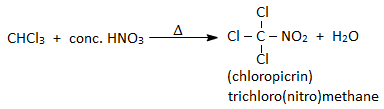
7. Reaction with HNO3 :
On heating with conc. HNO3, chloroform gives chloropicrin. Chloropicrin is used as an insecticide and tear gas.
8. Reduction :
Chloroform undergoes reduction with Zn/HCl to give methylene chloride but when treated with Zn/H2O (neutral medium) methane is obtained.
- DDT
- Picric acid
- BHC
- Urotropin
- Chloropicrin
- TNT
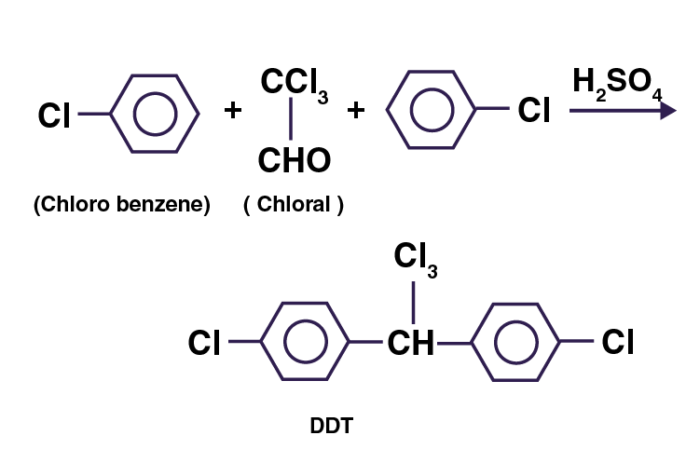
DDT is prepared by heating chloral and chlorobenzene in a 1:2 ratio in the presence of conc. sulphuric acid.
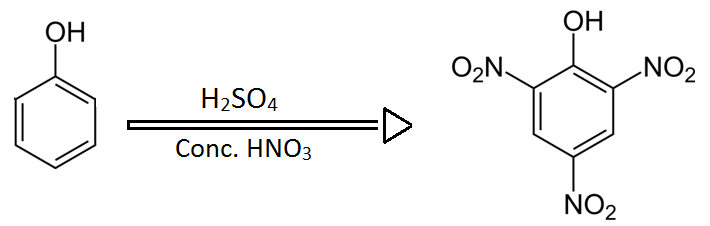
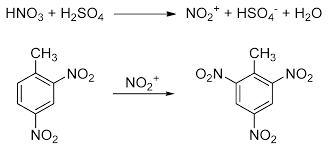
Picric acid is basically prepared from phenol by reacting phenol with concentrated sulphuric acid and concentrated nitric acid. The nitro groups from nitric acid attacks on ortho and para positions which is the best suitable and stable position for the newly formed compound which is picric acid.
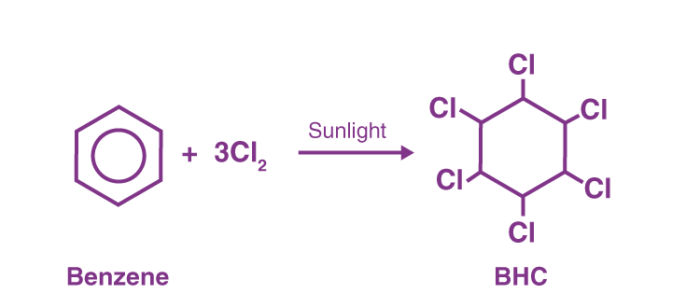
Chlorine combines with benzene, in the presence of sunlight and in the absence of oxygen as well as substitution catalysts, to form hexachlorocyclohexane.
Lindane can be prepared from chlorine and benzene by photochlorination. The product obtained i.e benzene hexachloride comprises isomers from which only the gamma-isomer is wanted. Gamma-isomer is got by treating the reaction mixture with acetic acid or methanol in which only the alpha and beta isomers dissolve easily.
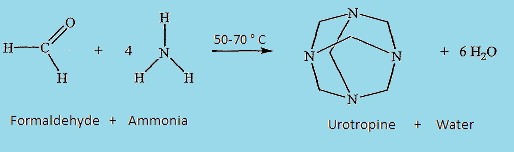
It is a nitrogenous compound, also known as hexamine with a cage-like structure similar to adamantane. It contains 4 molecules of nitrogen in each corner of its tetrahedral structure. It is used for various purposes in different industries like food additives, explosives, and many more.
Chloropicrin is prepared by heating chloroform with concentrated nitric acid.
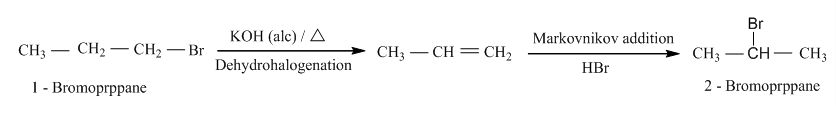
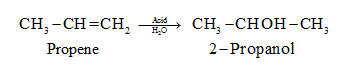
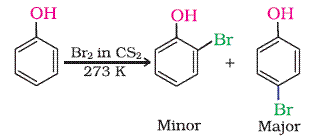
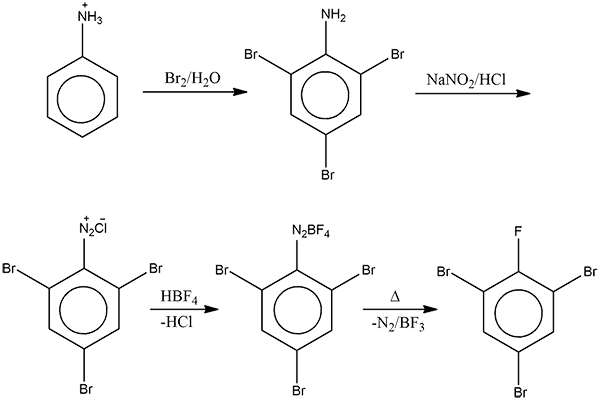
- 1-bromopropane to 2- bromopropane and vice versa.
- propan-1-ol to propan-2-ol and vice versa.
- Step 1
- Step 2
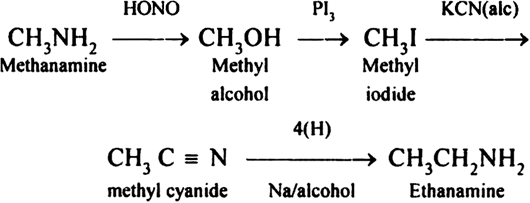
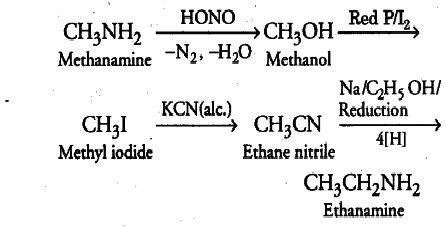
- Methanamine to ethanamine and vice versa.
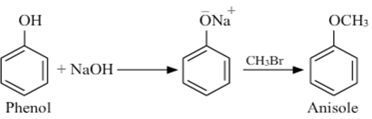
- Phenol to anisole(methoxy benzene) and vice versa.
- Ethoxy ethane to methoxy ethane.
- Phenol/aniline to azo-dye
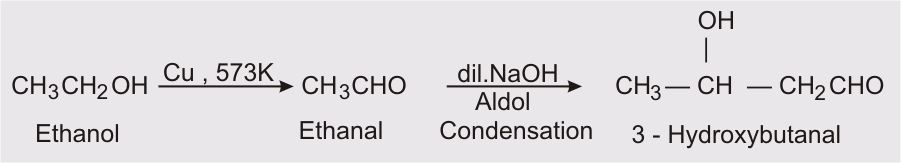
- Ethanal/ ethanol to 3-hydroxy butanal
- Ethanol to 2-hydroxyl propanoic acid
- Propanone(acetone) to 2-hydroxy-2-methyl propanoic acid
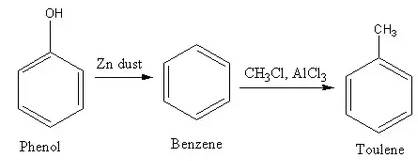
- Phenol to toluene
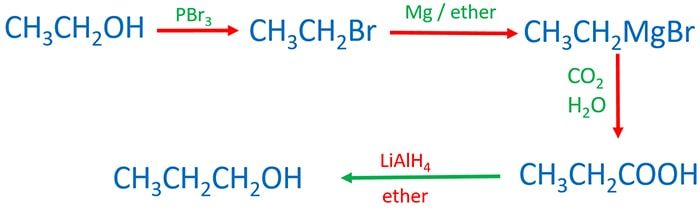
- Ethanol to propanol/ propanoic acid
- Methanamide to ethanamine
Ethanol on heating at 573K in presence of copper it converts into ethanal and then by aldol condensation we get 3-hydroxybutanal.
- Why is chloroform stored in a dark bottle containing ethanol?
- Why does chloroform not give white ppt with aq AgNO3?
- Why is nucleophilic substitution reaction difficult in haloarene?
- Why is the boiling point of ethanol greater than that of ethoxy ethane?
- Why is phenol more acidic than aliphatic alcohol?
- Why does nitrobenzene undergo an electrophilic substitution reaction at meta position? (Explain why -NO2 group is meta directing towards electrophilic aromatic substitution)
- Why is chlorobenzene o/p - directing towards electrophilic substitution reaction?
- It is dangerous to boil a sample of ether stored for a long time, give a reason.
- Ether is stored in a bottle containing iron wire, why?
- Give a suitable test to distinguish ethanamine from N-methyl methanamine.
- Write a chemical test to distinguish ethanoic acid(acetic acid) from methanoic acid(formic acid).
- Why is a chloroacetic acid stronger acid than acetic acid?
- Why is a formic acid stronger acid than acetic acid?
- Why an amino group of aniline is protected before nitration? (Aniline can not be nitrated directly, why?)
- Write the functional isomers of C3H80 with their IUPAC name. Give a chemical test to distinguish them.
- Write an unsymmetrical ether of C3H8O. How would you prepare this ether using Williamson's synthesis?
- Write down possible isomeric amines of C3H9N and give their IUPAC names.
- What is a peptide bond(linkage)? Write an example of the dipeptide.
- What are disaccharides? What happens when a disaccharide is hydrolyzed.
- Define protein. What is meant by denaturation of protein?
- What is soap? How is soap obtained from fat? (What is saponification?)(What happens when a fat/oil is hydrolyzed?)
- Write monomers and one use of:
- Bakelite
- Nylon-6,6
- Polyvinyl chloride(PVC)
- Teflon
- Analgesic drug antipyretic drug * Structural formula * Uses:-Analgesics are medications that relieve pain.
- Antibiotics Antiseptics * Structural formula * Uses:-Antibiotics that are effective against several different types of harmful microorganisms are called broad-spectrum antibiotics. eg. chloramphenicol, tetracycline, etc.
- Herbicide Pesticide
- Germicide Insecticide
- A synthetic fertilizer – nitrogen fertilizer/Phosphorus (phosphatic) fertilizer, mixed(NPK) fertilizer.
- Sodium benzoate is heated with soda lime.
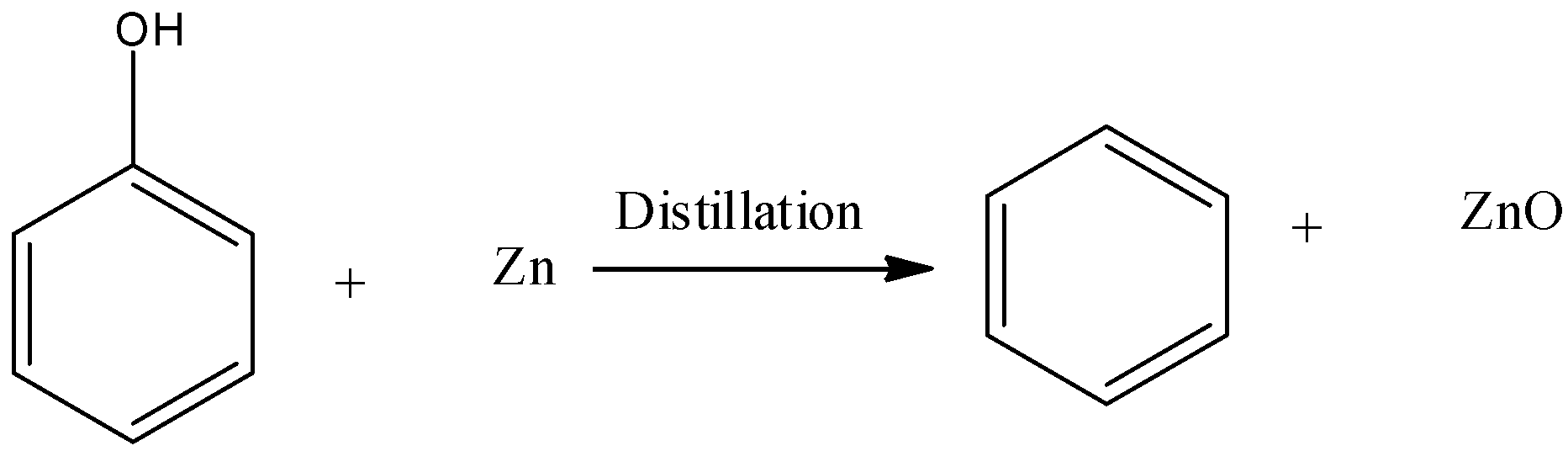
- Phenol is heated with zinc dust.
- Chlorobenzene is treated with chloral.
- Ethyl alcohol(ethanol) is treated with acetic acid(ethanoic acid)
- Phenol is treaded with aq. Br
- Aniline is treated with aq. Br
- Phenol is treated with benzene diazonium chloride.
- Ethoxy ethane is treated with HI.
- Methanal(formaldehyde) is treated with ammonia
- Methanal/benzaldehyde is treated with NaOH.
- Ethanal/propanone is treated with NaOH.
- Aldehyde/ketone is treated with hydroxylamine.
- Ethanol is heated with conc. H2SO
- Acetic acid is treated with P205
➵Chloroform is slowly oxidized by air in the presence of light to an extremely poisonous gas, carbonyl chloride, also known as phosgene. It is therefore stored in closed dark-colored bottles completely filled so that air is kept out.
➵Pure chloroform does not give a white precipitate with silver nitrate. That’s because chloroform does not contain chloride ions and it has just covalent bonds, non-ionizable carbon-chlorine bonds. It’s insoluble in water, but even if it were soluble, the carbon-chlorine bonds would not ionize. So, no AgCl precipitate.
➵In haloarenes C–X bond acquires a partial double bond character due to resonance. As a result, the bond cleavage in haloarenes is difficult than haloalkanes, and therefore, they are less reactive towards nucleophilic substitution reaction.
➵Ethanol has a higher boiling point than ethane due to intermolecular hydrogen bonding. Intermolecular hydrogen bonding is possible in ethanol but not possible in ethane. Intermolecular hydrogen bonding leads to molecular association and increases boiling point as energy is required to break these hydrogen bonds. Hydrogen bonding is possible when the H atom is attached to an electronegative N, O, or F atom.
➵Phenol is more acidic than aliphatic alcohols due to the stabilization of phenoxide ions through resonance. The presence of electron-withdrawing group increases the acidity of phenol by, stabilizing phenoxide ion while the presence of electron releasing group decreases the acidity of phenol by destabilizing phenoxide ion.
➵This is because the nitro group (−NO2) at o/p positions withdraw the electrons from the benzene ring which facilitates the attack of the nucleophile. The negative charge in the carbanion formed at o/p positions with respect to halogen atom is stabilized by the presence of nitro groups (−NO2) and resonance respectively.
➵Chlorine atoms attached to the ring exhibit dual nature. Chlorine atoms show an electron releasing nature due to the resonance effect. The lone pair present on the chlorine atom delocalizes itself with pi-electrons of the ring. The electron released into the ring stabilizes the positive charge at ortho and para positions due to the attack of an electrophile. Hence, the substitution of an electrophile is favored at ortho and para positions.
➵Due to the presence of lone pair of electrons on the ethereal oxygen, ether when comes in contact with atmospheric oxygen in the presence of sunlight, reacts with oxygen to form ether peroxide. Ether peroxide is highly unstable and explodes violently on heating causing serious accidents. Therefore, it is dangerous to boil the sample of ether stored for a long time.
➵Ether is highly reactive towards atmospheric oxygen in the presence of sunlight and forms peroxide which explodes on heating causing a serious accident.
When ether is kept in a bottle containing iron wire, the oxygen combines with iron to form iron oxide and it prevents the formation of peroxide. Therefore, ether is stored in a bottle containing iron wire.
➵Ethanamine and N-methylmethanamine can be distinguished by the carbylamines test. Ethanamine (i.e. primary amine) reacts with chloroform and alcoholic KOH to form ethyl isocyanide having an offensive smell. But N-methylmethanamine (i.e. secondary amine) does not give this test.
➵
➵Due to the presence of more electronegative atom Cl, the electron density on H of the carboxyl group of chloroacetic acid is lower compare to acetic acid and hence chloroacetic acid can release H an easier way.
➵Acetic acid contains an electron-donating methyl group. It destabilizes the conjugate base of acetic acid.
Formic acid has no such electron-donating group and is a stronger acid than acetic acid.
➵ The hurdles for the nitration of aniline are overcome by the protection of the amino groups by acetylation. The acetyl group reduces the reactivity of the ring and thus its oxidation does not occur easily with nitric acid HNO
➵
IUPAC name: isopropyl alcohol
➵
➵IUPAC Name:- propan-1-amine
There are four isomers of C3H9N.
They are propylamine
➵The molecules having peptide linkage are known as peptides. Peptide linkage is also known as a peptide bond. it is an amide formed between −COOH and −NH2 group by elimination of a water molecule. It is represented as
➵Disaccharides: Carbohydrates which on hydrolysis produce two molecules of monosaccharides of the same or different types are called disaccharides. For example sucrose, maltose, lactose, etc.
A disaccharide on hydrolysis gives two molecules of the same or one molecule each of two different monosaccharides since a disaccharide is defined as the sugar formed by the glycosidic linkage of 2 monosaccharides. Consider, for example, maltose. It hydrolyses to give 2 molecules of glucose.
➵Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues
On heating protein or on mixing concentrated acid or base its bioactivity gets destroyed. Protein gets coagulated to give a precipitate. This process is called the denaturation of protein.
➵Saponification is a process that involves the conversion of fat, oil, or lipid, into soap and alcohol by the action of aqueous alkali (e.g. NaOH).
➵Bakelite is a polymer that is made up of two monomers: phenol and formaldehyde.
*Uses
Bakelite due to its high resistance to electricity and heat is used in automotive components and industrial applications.
➵ There are two monomers in Nylon 6,6. These monomers are adipic acid and hexamethylenediamine.
*Uses
Nylon 6,6 fibers are used for clothing, carpets, tire cords, conveyor belts, and brushes
➵vinyl chloride is the monomer of Teflon.
*Uses
It is used for making bottles.
➵Tetrafluoroethane is monomer of teflon.
*Uses
It is used in making waterproof fabric.
* Structural formula * Uses:-It is directly applied to the infected parts i.e. they are usually of external use.
➵Sodium benzoate when heated with soda-lime it gets decarboxylated (removal of carbon dioxide) and benzene is obtained.
➵Phenol undergoes a reduction reaction when heated with Zinc dust and forms benzene. -It is one of the vital methods to prepare benzene.
➵ Chlorobenzene reacts readily with chlorine, nitric acid, or sulfuric acid, forming dichlorobenzenes, chloronitrobenzenes, or chlorobenzenesulfonic acids, respectively, and with chloral in the presence of sulfuric acid to form DDT, an insecticide.
➵When ethyl alcohol reacts with acetic acid, the hydroxyl group of the alcohol and the hydrogen of the acid react together and get eliminated as a water molecule. The ethyl (C2H5) groups of ethyl alcohol and acetic acid react together to form an ester. The ester, thus, formed is called ethyl ethanoate or ethyl acetate.
➵ When phenol reacts with bromine water 2,4,6- tribromophenol is formed. Bromine water is decolorized and a white precipitate is formed.
➵ When aniline is treated with bromine water, the bromine water gets decolorized, and a white precipitate is formed. This reaction results in the formation of 2,4,6-tribromo phenylamine. This reaction is an example of bromination.
➵Benzene diazonium chloride on reaction with phenol in a basic medium gives p-Hydroxy azobenzene. This is an example of a coupling reaction.
➵Ether reacts with hydroiodic acid to form alcohols and corresponding haloalkanes.
CH3CH2OCH2CH3 + HI→ CH3CH2I + CH3CH2OH
➵When formaldehyde is treated with ammonia, it forms urotropine. Write structural formulae and names of the four possible aldol condensation products from propanal and butanal.
➵This reaction is called Cannizaro's reaction. The Cannizzaro reaction is a redox reaction in which two molecules of an aldehyde are reacted to produce primary alcohol and a carboxylic acid using a hydroxide base.
➵ In presence of dil NaOH, two molecules of acetaldehyde add together to give βhydroxyaldehyde (aldol). The reaction is called aldol condensation.
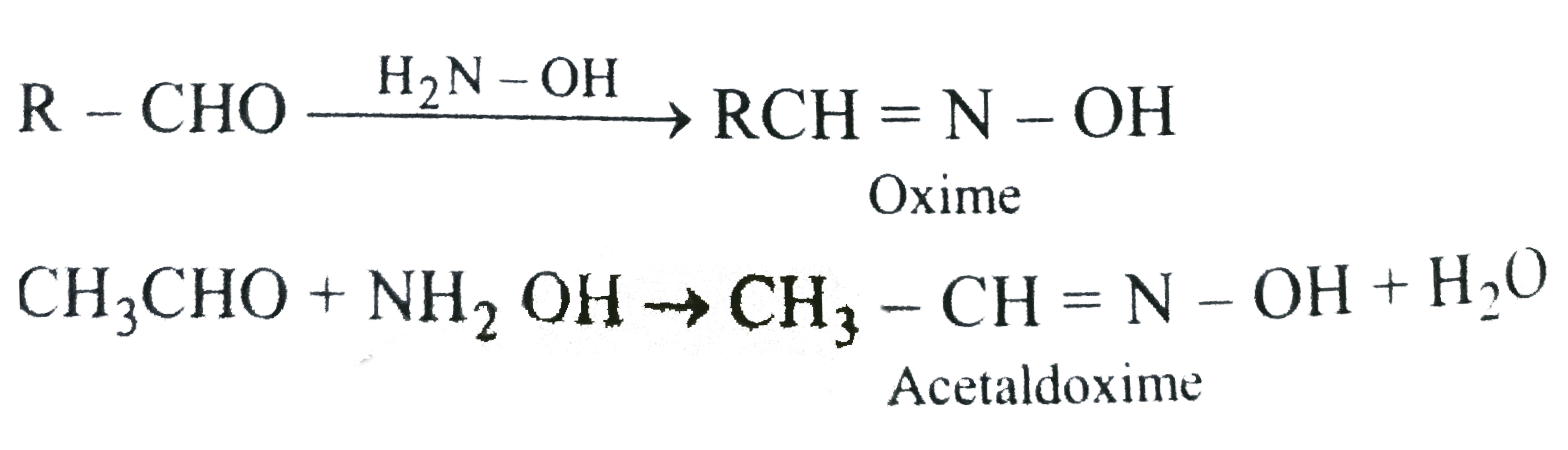
➵The reaction of aldehydes and ketones with hydroxylamine gives oximes.
➵When ethanol is heated with concentrated sulphuric acid at 170oC, it gets dehydrated to form ethene (unsaturated hydrocarbon). This reaction is termed a dehydration reaction as it involves the removal of water molecules from the alcohol (ethanol).
➵On heating Acetic acid with P2O5, it will form ethanoic anhydride. Acetic acid is the common name of ethanoic acid. The chemical formula of it is CH3-OOH. It is a carboxylic acid with two C atoms. P2O5 is phosphorus pentaoxide that is a very good dehydrating agent and involves in dehydration reactions of carboxylic acids mainly. Here when acetic acid is heated with P2O5, it will form ethanoic anhydride.
2CH3COOH + P2O5→ CH3-CO-O-CO-CH3 + H2O